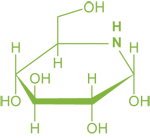
Molecule of D-glucose (Sugar)
Equivalent iminosugar, with ring oxygen replaced by nitrogen
Iminosugars are analogues of sugars in which the
oxygen is replaced by a
nitrogen atom. This substitution prevents normal metabolism resulting in inhibition of
glycosidases and
glycosyltransferases.
Iminosugars
The iminosugars are a widespread group of plant and microbial compounds that are attracting interest as therapeutic agents due to their ability to interact with human glycosidases,
other proteins and sugar receptors
[1]. They have many activities beneficial to health but due to difficulties with their identification and isolation most remain uninvestigated.
Carbohydrates are crucially important to the function of the human body and aberrations in sugar metabolism occur in most diseases; iminosugars can correct many of these faults.
In their simplest form they resemble furanose and pyranose monosaccharides with a nitrogen replacing the oxygen in the ring (see image).
The five ring structures fitting this classification that are most common in nature are: pyrrolidine, piperidine, pyrrolizidine, indolizidine and nor-tropane.
In the past, these compounds have been referred to by several names, including aza-sugars, glycosidase inhibitors and sugar analogues, and the variety and distribution of natural compounds
have been reviewed
[2,3]. Their activities on glycosidases can be very potent and precise unlike many non-specific glycosidase inhibitors that will bind to many proteins and, therefore,
be inactivated before reaching their target enzymes.
Where they are found
The iminosugars seem to be more or less ubiquitous in plants, although there are taxonomic patterns in their distribution, e.g. calystegines occur in almost all species of the
Solanaceae except in the genus Nicotiana but are rarely found in other plant families. The analysis, isolation and identification of iminosugars is difficult and has only been mastered
by a limited number of researchers (Nash, Asano, Kato, Fleet and Molyneux have jointly reported most of the natural compounds
[2,4]).
Not all plants accumulate high concentrations of iminosugars and the amounts present can be below normal analytical detection levels.
They may also be common microbial products but very few organisms have been studied due to difficulties in analysis.
References
- Nash RJ, Kato A, Yu C-Y, Fleet GWJ (2011) Iminosugars as therapeutic agents: recent advances and promising trends. Future Med. Chem. 3 (12): 1513-1521.
- Watson AA, Fleet GWJ, Asano N, Molyneux RJ, Nash RJ (2001) Polyhydroxylated alkaloids — natural occurrence and therapeutic applications. Phytochemistry 56, 265-295.
- Asano N, Nash RJ, Molyneux RJ, Fleet GWJ (2000). Nitrogen-in-the-Ring Sugar Mimetics: Natural Occurrence, Biological Activity and Prospects for Therapeutic Application. Tetrahedron Asymmetry 11: 1645-1680.
- Kato A, Kato N, Adachi I, Hollinshead J, Fleet GWJ, Kuriyama C, Ikeda K, Asano N, Nash RJ (2007) Isolation of glycosidase-inhibiting hyacinthacines and related alkaloids from Scilla socialis, J. Nat. Prod: 70: 993-997.
- Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, Cotman C, Cottrell B, Montine TJ, Thomas RG, Aisen P (2012) Antioxidants for Alzheimer Disease: A Randomized Clinical Trial With Cerebrospinal Fluid Biomarker Measures. Arch Neurol. 69: 836-841.
- Thompson AL, Michalik A, Nash RJ, Wilson FX, van Well R, Johnson P, Fleet GWJ, Yu C-Y, Hu X-G, Cooper RI and Watkin DJ (2009) Steviamine, a new class of indolizidine alkaloid [(1R,2S,3R,5R,8aR)-3-(Hydroxymethyl)-5- methyloctahydroindolizine-1,2-diol hydrobromide] Acta Cryst. E65, o2904.